Welcome To TAMIU Student Health
If you are a registered student in need of an appointment, call 956.326.2235. You can also contact us at studenthealth@tamiu.edu.
Hours of Operation
Monday and Tuesday: 8 a.m. - 5 p.m.
Wednesday and Thursday: 8 a.m. - 6 p.m.
Friday: 8 a.m. - 3 p.m.
Closed on Saturday and Sunday
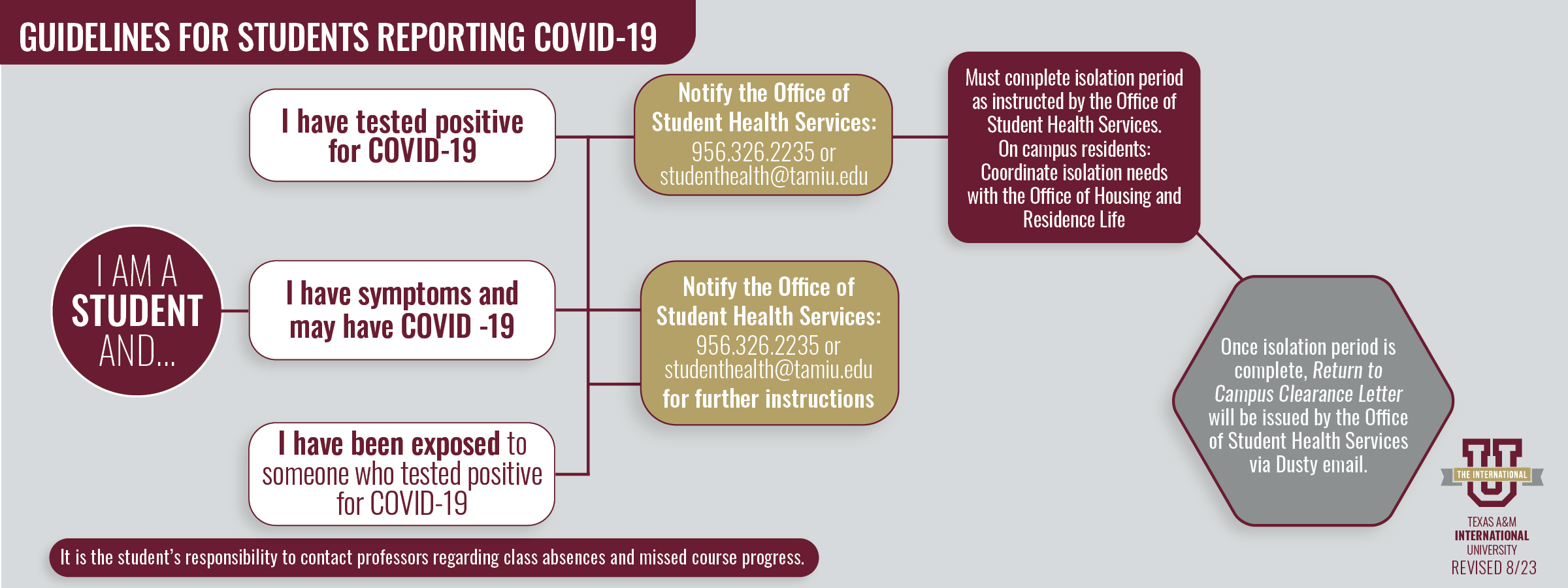
COVID-19 reporting information for enrolled students
If you have tested positive for COVID-19 and are experiencing symptoms, call the Office of Student Health Services for further guidance. You can reach us at 956.326.2235.
Rapid Antigen Testing
(Available ONLY to registered TAMIU students)
BE ADVISED: COVID-19 ANTIGEN TESTING IS AVAILABLE FOR ENROLLED STUDENTS AT THE OFFICE OF STUDENT HEALTH SERVICES.
FOR MORE INFORMATION OR TO SCHEDULE AN APPOINTMENT, PLEASE CALL 956.326.2235.
CDC recommends the 2023-2024 updated COVID-19 vaccines:
- Pfizer-BioNTech and Moderna or Novavax to protect against serious illness from COVID-19..
- There is no preferential recommendation for the use of any one COVID-19 vaccine over another when more than one licensed or authorized, recommended, and age-appropriate vaccine is available.
Everyone aged 5 years and older should get 1 dose of updated COVID-19 vaccine to protect against serious illness form COVID-19
People aged 12 years and older who got Pfizer-BioNTech or Moderna COVID-19 vaccines before September 12, 2023, or Novavax COVID-19 vaccine before October 3, 2023, should get 1 updated Pfizer-BioNTech, Moderna, or Novavax COVID-19 vaccine.
People who are moderately or severely immunocompromised may get additional doses of COVID-19 vaccine
People aged 65 years and older who received 1 dose of any updated 2023-2024 COVID-19 vaccine (Pfize-BioNTech, Moderna or Novovax) should receive 1 additional dose of an updated COVID-19 vaccine at least 4 months after the previous updated dose.
COVID-19 VACCINE OPPORTUNITY ON CAMPUS
Student Health Services is proud to collaborate with Gateway Community Health Center to offer updated COVID-19 vaccines on campus for any TAMIU student or employee.
*STAY TUNED FOR FUTURE OPPORTUNITIES*
In addition, multiple locations in our community may offer updated COVID-19 immunizations. Please call to verify vaccine availability:
City of Laredo Health Department
2600 Cedar
956.795.4906
Gateway Community Health Center
1515 Pappas Street
956.795.8100
Recommendation for Everyone Aged 5 Years and Older
- CDC recommends the 2023-2024 updated COVID-19 vaccines.
- Everyone 5 years and older should get 1 updated Pfizer-BioNTech or Moderna COVID-19 vaccine, to protect against serious illness from COVID-19.
- People aged 12 years and older who got Pfizer-BioNTech or Moderna COVID-19 vaccines before September 12, 2023, or Novavax COVID-19 vaccine before October 3, 2023, should get 1 updated Pfizer-BioNTech, Moderna, or Novavax COVID-19 vaccine.
- COVID-19 vaccine recommendations will be updated as needed.
Recommendation for People Who May Get Additional Updated COVID-19 Vaccines
- Some people may get additional doses of COVID-19 vaccines:
- People who are moderately or severely immunocompromised may get 1 additional updated COVID-19 vaccine dose 2 or more months after the last recommended updated COVID-19 vaccine. The additional dose(s) help your immune system to better protect you against COVID-19 infection. Talk to your healthcare provider about additional updated doses.
- People aged 65 years and older who received 1 dose of any updated 2023-2024 COVID-19 vaccine (Pfize-BioNTech, Moderna or Novovax) should receive 1 additional dose of an updated COVID-19 vaccine at least 4 months after the previous updated dose.
Getting Vaccines If you Had or Currently Have COVID-19
If you recently had COVID-19, you still need to stay up to date with your vaccines, but you may consider delaying your next vaccine dose by 3 months.
Reinfection is less likely in the weeks to months after infection. However, certain factors could be reasons to get a vaccine sooner than later, such as:
- personal risk of severe disease,
- risk of disease in a family or household member or other close contact,
- local levels of COVID-19 in your area,
- and the most common COVID-19 variant currently causing illness.
What You Need To Know
- COVID-19 hospital admission levels can help individuals and communities decide which prevention actions they can take based on the latest information.
- For each level, CDC recommends actions you can take to help protect yourself and others from severe impacts of COVID-19.